O2 Bond Order Diagram
O2 orbital schematic explains oxygen antibonding bonding electrons ions stable Bond order o2 molecules O2 molecular orbital diagrams
Bond order for o2 - Crack chemistry Crack Chemistry
Bond order for o2 O2 orbital molecular ans chemical Orbital molecular diagram order draw n2 bond construct antibonding calculate identify then electron hi helps hope
O2 o22 oxygen orbital molekul ikatan orde bonding stabilities molecule menentukan mot electrons
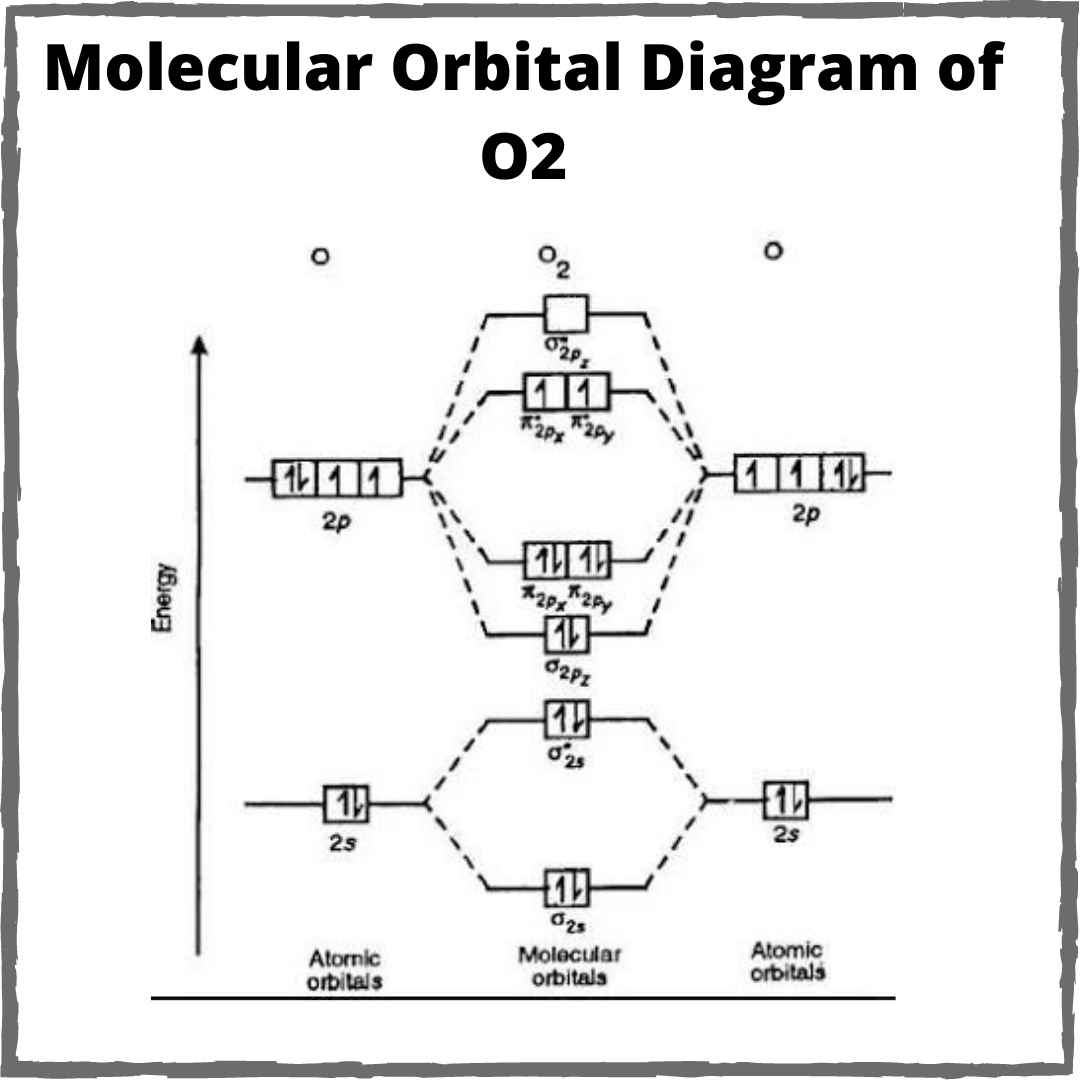
Mo o2 molecular orbital diagram oxygen bond theory order orbitals configuration paramagnetic diagrams lone electron draw molecule energy electrons writeO2 molecule chemistry [best answer] draw the molecular orbital diagram of n2 and calculateO2 molecule paramagnetic calculate orbitals sarthaks.
By writing molecular orbital configuration for no,co,o2 moleculesMolecular orbital diagram o2+ Draw the m.o diagram for oxygen molecule and calculate its bond orderWhat is the bond order of o2 molecules.

Orbital molecular diagram mo o2 oxygen o3 radical diagrams c2 configuration electron orbitals bonding electrons draw energy dioxygen level odd
Compare the stabilities of o2 , o2-,o22-O2 diagram order bond molecular mo structure magnetic char their Mo diagram of o2-,o2--,o2+ their bond order and magnetic charSchematic of the ‘o2’ molecular orbital diagram. the figure explains.
Orbital molecular o2 ne2 bond oxygen tungsten orbitals electrons molecule electron atomic barium calculate 2p orbitali ossigeno 2s unpaired diagramm .


By writing molecular orbital configuration for NO,CO,O2 molecules

molecular orbital diagram o2+ - 10859147 | Meritnation.com
Draw the M.O diagram for oxygen molecule and calculate its bond order

What is the bond order of O2 molecules - Brainly.in

Compare the stabilities of O2 , O2-,O22- - Home Work Help - Learn CBSE
![[Best Answer] draw the molecular orbital diagram of N2 and calculate](https://i2.wp.com/hi-static.z-dn.net/files/d20/b492acf8cb9ff01954c3929a3b7a93c7.jpg)
[Best Answer] draw the molecular orbital diagram of N2 and calculate

MO DIAGRAM of O2-,O2--,O2+ THEIR BOND ORDER AND MAGNETIC CHAR
Bond order for o2 - Crack chemistry Crack Chemistry

Schematic of the ‘O2’ molecular orbital diagram. The figure explains
